Active Transport
Active transport (or active uptake) is the mediated transport of biochemicals, and other atomic/molecular substances, across membranes. Unlike passive transport, this process requires the expenditure of cellular energy to move molecules "uphill" against a gradient.
ProcessIn this form of transport, molecules move against either an electrical or concentration gradient (collectively termed an electrochemical gradient).
- The active transport of small molecules or ions across a cell membrane is generally carried out by transport proteins that are found in the membrane.
- Larger molecules such as starch can also be actively transported across the cell membrane by processes known as endocytosis and exocytosis.
- The process whereby particles are moved through a membrane from a region of low concentration to a region of high concentration is known as active transport
Relation to Cellular Energy
Using conservative assumptions, it has been calculated that the amount of energy available to the cell is insufficient to power the estimated degree of active transport, e.g. by a factor of 15-30 times for the sodium pump alone . Following vigorous debate in Science and elsewhere, this discrepancy has yet to be resolved.
Plasmid DNA Extraction

Plasmid is a DNA molecule that is separate from, and can replicate independently of, the chromosomal DNA.[1] They are double stranded and, in many cases, circular. Plasmids usually occur naturally in bacteria, but are sometimes found in eukaryotic organisms .
Plasmids are often used to purify a specific sequence, since they can easily be purified away from the rest of the genome. For their use as vectors, and for molecular cloning, plasmids often need to be isolated.
There are several methods to isolate plasmid DNA from bacteria, the archetypes of which are the miniprep and the maxiprep/bulkprep.The former can be used to quickly find out whether the plasmid is correct in any of several bacterial clones. The yield is a small amount of impure plasmid DNA, which is sufficient for analysis by restriction digest and for some cloning techniques.

In the latter, much larger volumes of bacterial suspension are grown from which a maxi-prep can be performed. Essentially this is a scaled-up miniprep followed by additional purification. This results in relatively large amounts (several micrograms) of very pure plasmid DNA.
Multiple Sclerosis Animation
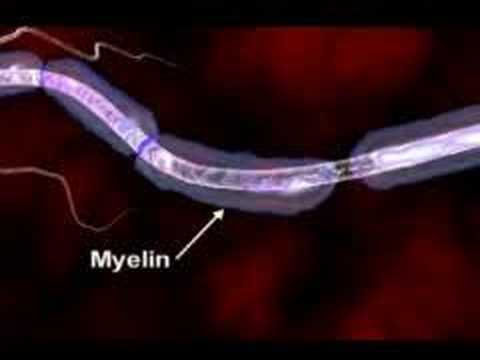
Multiple sclerosis (abbreviated MS, also known as disseminated sclerosis or encephalomyelitis disseminata) is an autoimmune condition in which the immune system attacks the central nervous system, leading to demyelination. Disease onset usually occurs in young adults, and it is more common in women. It has a prevalence that ranges between 2 and 150 per 100,000. MS was first described in 1868 by Jean-Martin Charcot.
MS affects the ability of nerve cells in the brain and spinal cord to communicate with each other. Nerve cells communicate by sending electrical signals called action potentials down long fibers called axons, which are wrapped in an insulating substance called myelin. In MS, the body's own immune system attacks and damages the myelin. When myelin is lost, the axons can no longer effectively conduct signals. The name multiple sclerosis refers to scars (scleroses – better known as plaques or lesions) in the white matter of the brain and spinal cord, which is mainly composed of myelin.Although much is known about the mechanisms involved in the disease process, the cause remains unknown. Theories include genetics or infections. Different environmental risk factors have also been found.
Multiple sclerosis (MS) is a disease that affects the central nervous system. the CNS which consist of the brain spinal cord optic nerves everything we do whether it's taking a step to solving a problem or simply breathing relies on the proper functioning of the CNS.To understand how MS may impact the CNS we must explore the disease at the cellular level .In the brain millions of nerve cells called neurons continually send and receive signals, each signal is a minute but necessary part of the intricate CNS orchestrations that culminate in the actions, sensations, thoughts and emotions that comprised the human experience.Normally the path over which a nerve signal travels is protected by a type of insulation called the myelin sheath,this insulation is essential for nerve signals to reach their target.In MS the myelin sheath is eroded and the underlying wire like nerve fiber is also damaged, this leads to a breakdown in the ability of the nerve cells transmit signals. it is believed that the loss of myelin is the result of mistaken attack of immune cells.Immune cells protect the body against foreign substances such as bacteria and viruses .But in MS something goes awry.Immune cells infiltrate the brain and spinal cord seek and attack.As ongoing inflammation and tissue damage occurs nerve signals are disrupted. this causes unpredictable symptoms that can range from numbness or tingling to blindness and paralysis.These losses may be temporary or permanent
DNA Extraction
DNA extraction is a routine procedure to collect DNA for subsequent molecular or forensic analysis. There are three basic steps in a DNA extraction:
- Breaking the cells open to expose the DNA within, such as by grinding or sonicating the sample.
- Removing membrane lipids by adding a detergent.
- Precipitating the DNA with an alcohol — usually ethanol or isopropanol. Since DNA is insoluble in these alcohols, it will aggregate together, giving a pellet on centrifugation. This step also removes alcohol-soluble salt.
Refinements of the technique include adding a chelating agent to sequester divalent cations such as Mg2+ and Ca2+. This stops dnase enzymes from degrading the DNA.
Cellular and histone proteins bound to the DNA can be removed prior to its precipitation either by adding a protease or having prior to precipitation, precipitating with sodium or ammonium acetate, or extracting with a phenol-chloroform mixture.
If desired, the DNA can be resolubilized in a slightly alkaline buffer.
Detecting DNA
A diphenylamine (DPA) indicators will confirm the presence of DNA. This procedure involves chemical hydrolysis of DNA: when heated (e.g. ≥95oC) in acid, the reaction requires a deoxyribose sugar and therefore is specific for DNA. Under these conditions, the 2-deoxyribose is converted to w-hydroxylevulinyl aldehyde, which reacts with the compound, diphenylamine, to produce a blue-colored compound. DNA concentration can be determined measuring the intensity of absorbance of the solution at the 600 nm with a spectrophotometer and comparing to a standard curve of known DNA concentrations.
Measuring the intensity of absorbance of the DNA solution at wavelengths 260 nm and 280nm is used as a measure of DNA purity. DNA absorbs UV light at 260 and 280 nm, and aromatic proteins absorbs UV light at 280 nm; a pure sample of DNA has the 260/280 ratio at 1.8 and is relatively free from protein contamination. A DNA preparation that is contaminated with protein will have a 260/280 ratio lower than 1.8.
DNA can be quantified by cutting the DNA with a restriction enzyme, running it on an agarose gel, staining with ethidium bromide or a different stain and comparing the intensity of the DNA with a DNA marker of known concentration.
Using the Southern blot technique this quantified DNA can be isolated and examined further using PCR and RFLP analysis. These procedures allow differentiation of the repeated sequences within the genome. It is these techniques which forensic scientists use for comparison and identification.
Western Blot Animation

The western blot (alternately, immunoblot) is a method of detecting specific proteins in a given sample of tissue homogenate or extract. It uses gel electrophoresis to separate native or denatured proteins by the length of the polypeptide (denaturing conditions) or by the 3-D structure of the protein (native/ non-denaturing conditions). The proteins are then transferred to a membrane (typically nitrocellulose or PVDF), where they are probed (detected) using antibodies specific to the target protein. There are now many reagent companies that specialize in providing antibodies (both monoclonal and polyclonal antibodies) against many thousands of different proteins. Commercial antibodies can be expensive, though the unbound antibody can be reused between experiments. This method is used in the fields of molecular biology, biochemistry, immunogenetics and other mo
Molecular biology disciplines.

Other related techniques include using antibodies to detect proteins in tissues and cells by immunostaining and enzyme-linked immunosorbent assay (ELISA). The method originated from the laboratory of George Stark at Stanford. The name western blot was given to the technique by W. Neal Burnette and is a play on the name Southern blot, a technique for DNA detection developed earlier by Edwin Southern. Detection of RNA is termed northern blotting.
Steps in a Western blot Tissue preparation
Samples may be taken from whole tissue or from cell culture. In most cases, solid tissues are first broken down mechanically using a blender (for larger sample volumes), using a homogenizer (smaller volumes), or by sonication. Cells may also be broken open by one of the above mechanical methods. However, it should be noted that bacteria, virus or environmental samples can be the source of protein and thus Western blotting is not restricted to cellular studies only. Assorted detergents, salts, and buffers may be employed to encourage lysis of cells and to solubilize proteins. Protease and phosphatase inhibitors are often added to prevent the digestion of the sample by its own enzymes. A combination of biochemical and mechanical techniques – including various types of filtration and centrifugation – can be used to separate different cell compartments and organelles.
Gel electrophoresis
The proteins of the sample are separated using gel electrophoresis. Separation of proteins may be by isoelectric point (pI), molecular weight, electric charge, or a combination of these factors. The nature of the separation depends on the treatment of the sample and the nature of the gel. By far the most common type of gel electrophoresis employs polyacrylamide gels and buffers loaded with sodium dodecyl sulfate (SDS). SDS-PAGE (SDS polyacrylamide gel electrophoresis) maintains polypeptides in a denatured state once they have been treated with strong reducing agents to remove secondary and tertiary structure (e.g. S-S disulfide bonds to SH and SH) and thus allows separation of proteins by their molecular weight. Sampled proteins become covered in the negatively charged SDS and move to the positively charged electrode through the acrylamide mesh of the gel. Smaller proteins migrate faster through this mesh and the proteins are thus separated according to size (usually measured in kilo Daltons, kD). The concentration of acrylamide determines the resolution of the gel - the greater the acrylamide concentration the better the resolution of lower molecular weight proteins. The lower the acrylamide concentration the better the resolution of higher molecular weight proteins. Proteins travel only in one dimension along the gel for most blots. Samples are loaded into wells in the gel. One lane is usually reserved for a marker or ladder, a commercially available mixture of proteins having defined molecular weights, typically stained so as to form visible, coloured bands. An example of a ladder is the GE Full Range Molecular weight ladder (Figure 1). When voltage is applied along the gel, proteins migrate into it at different speeds. These different rates of advancement (different electrophoretic mobilities) separate into bands within each lane. It is also possible to use a two-dimensional (2-D) gel which spreads the proteins from a single sample out in two dimensions. Proteins are separated according to isoelectric point (pH at which they have neutral net charge) in the first dimension, and according to their molecular weight in the second dimension.
Transfer
In order to make the proteins accessible to antibody detection, they are moved from within the gel onto a membrane made of nitrocellulose or polyvinylidene fluoride (PVDF). The membrane is placed on top of the gel, and a stack of tissue papers placed on top of that. The entire stack is placed in a buffer solution which moves up the paper by capillary action, bringing the proteins with it. Another method for transferring the proteins is called electroblotting and uses an electric current to pull proteins from the gel into the PVDF or nitrocellulose membrane. The proteins move from within the gel onto the membrane while maintaining the organization they had within the gel. As a result of this "blotting" process, the proteins are exposed on a thin surface layer for detection (see below). Both varieties of membrane are chosen for their non-specific protein binding properties (i.e. binds all proteins equally well). Protein binding is based upon hydrophobic interactions, as well as charged interactions between the membrane and protein. Nitrocellulose membranes are cheaper than PVDF, but are far more fragile and do not stand up well to repeated probings. The uniformity and overall effectiveness of transfer of protein from the gel to the membrane can be checked by staining the membrane with Coomassie or Ponceau S dyes. Coomassie is the more sensitive of the two, although Ponceau S's water solubility makes it easier to subsequently destain and probe the membrane as described below.
Blocking
Since the membrane has been chosen for its ability to bind protein, and both antibodies and the target are proteins, steps must be taken to prevent interactions between the membrane and the antibody used for detection of the target protein. Blocking of non-specific binding is achieved by placing the membrane in a dilute solution of protein - typically Bovine serum albumin (BSA) or non-fat dry milk (both are inexpensive), with a minute percentage of detergent such as Tween 20. The protein in the dilute solution attaches to the membrane in all places where the target proteins have not attached. Thus, when the antibody is added, there is no room on the membrane for it to attach other than on the binding sites of the specific target protein. This reduces "noise" in the final product of the Western blot, leading to clearer results, and eliminates false positives.
Detection
During the detection process the membrane is "probed" for the protein of interest with a modified antibody which is linked to a reporter enzyme, which when exposed to an appropriate substrate drives a colourimetric reaction and produces a colour. For a variety of reasons, this traditionally takes place in a two-step process, although there are now one-step detection methods available for certain applications.
Two step
Primary antibody
Antibodies are generated when a host species or immune cell culture is exposed to the protein of interest (or a part thereof). Normally, this is part of the immune response, whereas here they are harvested and used as sensitive and specific detection tools that bind the protein directly. After blocking, a dilute solution of primary antibody (generally between 0.5 and 5 micrograms/ml) is incubated with the membrane under gentle agitation. Typically, the solution is composed of buffered saline solution with a small percentage of detergent, and sometimes with powdered milk or BSA. The antibody solution and the membrane can be sealed and incubated together for anywhere from 30 minutes to overnight. It can also be incubated at different temperatures, with warmer temperatures being associated with more binding, both specific (to the target protein, the "signal") and non-specific ("noise").
Secondary Antibody
After rinsing the membrane to remove unbound primary antibody, the membrane is exposed to another antibody, directed at a species-specific portion of the primary antibody. This is known as a secondary antibody, and due to its targeting properties, tends to be referred to as "anti-mouse," "anti-goat," etc. Antibodies come from animal sources (or animal sourced hybridoma cultures); an anti-mouse secondary will bind to just about any mouse-sourced primary antibody. This allows some cost savings by allowing an entire lab to share a single source of mass-produced antibody, and provides far more consistent results. The secondary antibody is usually linked to biotin or to a reporter enzyme such as alkaline phosphatase or horseradish peroxidase. This means that several secondary antibodies will bind to one primary antibody and enhances the signal. Most commonly, a horseradish peroxidase-linked secondary is used in conjunction with a chemiluminescent agent, and the reaction product produces luminescence in proportion to the amount of protein. A sensitive sheet of photographic film is placed against the membrane, and exposure to the light from the reaction creates an image of the antibodies bound to the blot. As with the ELISPOT and ELISA procedures, the enzyme can be provided with a substrate molecule that will be converted by the enzyme to a colored reaction product that will be visible on the membrane . A third alternative is to use a radioactive label rather than an enzyme coupled to the secondary antibody, such as labeling an antibody-binding protein like Staphylococcus Protein A with a radioactive isotope of iodine. Since other methods are safer, quicker and cheaper this method is now rarely used.
One step
Historically, the probing process was performed in two steps because of the relative ease of producing primary and secondary antibodies in separate processes. This gives researchers and corporations huge advantages in terms of flexibility, and adds an amplification step to the detection process. Given the advent of high-throughput protein analysis and lower limits of detection, however, there has been interest in developing one-step probing systems that would allow the process to occur faster and with less consumables. This requires a probe antibody which both recognizes the protein of interest and contains a detectable label, probes which are often available for known protein tags. The primary probe is incubated with the membrane in a manner similar to that for the primary antibody in a two-step process, and then is ready for direct detection after a series of wash steps.
Analysis
After the unbound probes are washed away, the Western blot is ready for detection of the probes that are labeled and bound to the protein of interest. In practical terms, not all Westerns reveal protein only at one band in a membrane. Size approximations are taken by comparing the stained bands to that of the marker or ladder loaded during electrophoresis. The process is repeated for a structural protein, such as actin or tubulin, that should not change between samples. The amount of target protein is indexed to the structural protein to control between groups. This practice ensures correction for the amount of total protein on the membrane in case of errors or incomplete transfers.
Colorimetric detection
The colorimetric detection method depends on incubation of the Western blot with a substrate that reacts with the reporter enzyme (such as peroxidase) that is bound to the secondary antibody. This converts the soluble dye into an insoluble form of a different color that precipitates next to the enzyme and thereby stains the membrane. Development of the blot is then stopped by washing away the soluble dye. Protein levels are evaluated through densitometry (how intense the stain is) or spectrophotometry.
Chemiluminescence
Chemiluminescent detection methods depend on incubation of the Western blot with a substrate that will luminesce when exposed to the reporter on the secondary antibody. The light is then detected by photographic film, and more recently by CCD cameras which captures a digital image of the Western blot. The image is analysed by densitometry, which evaluates the relative amount of protein staining and quantifies the results in terms of optical density. Newer software allows further data analysis such as molecular weight analysis if appropriate standards are used. So-called "enhanced chemiluminescent" (ECL) detection is considered to be among the most sensitive detection methods for blotting analysis.
Radioactive detection
Radioactive labels do not require enzyme substrates, but rather allow the placement of medical X-ray film directly against the Western blot which develops as it is exposed to the label and creates dark regions which correspond to the protein bands of interest . The importance of radioactive detections methods is declining, because it is very expensive, health and safety risks are high and ECL provides a useful alternative.
Fluorescent detection
The fluorescently labeled probe is excited by light and the emission of the excitation is then detected by a photosensor such as CCD camera equipped with appropriate emission filters which captures a digital image of the Western blot and allows further data analysis such as molecular weight analysis and a quantitative Western blot analysis. Fluorescence is considered to be among the most sensitive detection methods for blotting analysis.
Secondary probing
One major difference between nitrocellulose and PVDF membranes relates to the ability of each to support "stripping" antibodies off and reusing the membrane for subsequent antibody probes. While there are well-established protocols available for stripping nitrocellulose membranes, the sturdier PVDF allows for easier stripping, and for more reuse before background noise limits experiments. Another difference is that, unlike nitrocellulose, PVDF must be soaked in 95% ethanol, isopropanol or methanol before use. PVDF membranes also tend to be thicker and more resistant to damage during use.
2-D Gel Electrophoresis
2-dimensional SDS-PAGE uses the principles and techniques outlined above. 2-D SDS-PAGE, as the name suggests, involves the migration of polypeptides in 2 dimensions. For example, in the first dimension polypeptides are separated according to isoelectric point, while in the second dimension polypeptides are separated according to their molecular weight. The isoelectric point of a given protein is determined by the relative number of positively (e.g. lysine and arginine) and negatively (e.g. glutamate and aspartate) charged amino acids, with negatively charged amino acids contributing to a high isoelectric point and positively charged amino acids contributing to a low isoelectric point. Samples could also be separated first under nonreducing conditions using SDS-PAGE and under reducing conditions in the second dimension, which breaks apart disulfide bonds that hold subunits together. SDS-PAGE might also be coupled with urea-PAGE for a 2-dimensional gel. In principle, this method allows for the separation of all cellular proteins on a single large gel. A major advantage of this method is that it often distinguishes between different isoforms of a particular protein - e.g. a protein that has been phosphorylated (by addition of a negatively charged group). Proteins that have been separated can be cut out of the gel and then analysed by mass spectrometry, which identifies the protein.
Medical diagnostic applications
The confirmatory HIV test employs a Western blot to detect anti-HIV antibody in a human serum sample. Proteins from known HIV-infected cells are separated and blotted on a membrane as above. Then, the serum to be tested is applied in the primary antibody incubation step; free antibody is washed away, and a secondary anti-human antibody linked to an enzyme signal is added. The stained bands then indicate the proteins to which the patient's serum contains antibody. A Western blot is also used as the definitive test for Bovine spongiform encephalopathy (BSE, commonly referred to as 'mad cow disease'). Some forms of Lyme disease testing employ Western blotting.
Molecular biology disciplines.
Other related techniques include using antibodies to detect proteins in tissues and cells by immunostaining and enzyme-linked immunosorbent assay (ELISA). The method originated from the laboratory of George Stark at Stanford. The name western blot was given to the technique by W. Neal Burnette and is a play on the name Southern blot, a technique for DNA detection developed earlier by Edwin Southern. Detection of RNA is termed northern blotting.
Steps in a Western blot Tissue preparation
Samples may be taken from whole tissue or from cell culture. In most cases, solid tissues are first broken down mechanically using a blender (for larger sample volumes), using a homogenizer (smaller volumes), or by sonication. Cells may also be broken open by one of the above mechanical methods. However, it should be noted that bacteria, virus or environmental samples can be the source of protein and thus Western blotting is not restricted to cellular studies only. Assorted detergents, salts, and buffers may be employed to encourage lysis of cells and to solubilize proteins. Protease and phosphatase inhibitors are often added to prevent the digestion of the sample by its own enzymes. A combination of biochemical and mechanical techniques – including various types of filtration and centrifugation – can be used to separate different cell compartments and organelles.
Gel electrophoresis
The proteins of the sample are separated using gel electrophoresis. Separation of proteins may be by isoelectric point (pI), molecular weight, electric charge, or a combination of these factors. The nature of the separation depends on the treatment of the sample and the nature of the gel. By far the most common type of gel electrophoresis employs polyacrylamide gels and buffers loaded with sodium dodecyl sulfate (SDS). SDS-PAGE (SDS polyacrylamide gel electrophoresis) maintains polypeptides in a denatured state once they have been treated with strong reducing agents to remove secondary and tertiary structure (e.g. S-S disulfide bonds to SH and SH) and thus allows separation of proteins by their molecular weight. Sampled proteins become covered in the negatively charged SDS and move to the positively charged electrode through the acrylamide mesh of the gel. Smaller proteins migrate faster through this mesh and the proteins are thus separated according to size (usually measured in kilo Daltons, kD). The concentration of acrylamide determines the resolution of the gel - the greater the acrylamide concentration the better the resolution of lower molecular weight proteins. The lower the acrylamide concentration the better the resolution of higher molecular weight proteins. Proteins travel only in one dimension along the gel for most blots. Samples are loaded into wells in the gel. One lane is usually reserved for a marker or ladder, a commercially available mixture of proteins having defined molecular weights, typically stained so as to form visible, coloured bands. An example of a ladder is the GE Full Range Molecular weight ladder (Figure 1). When voltage is applied along the gel, proteins migrate into it at different speeds. These different rates of advancement (different electrophoretic mobilities) separate into bands within each lane. It is also possible to use a two-dimensional (2-D) gel which spreads the proteins from a single sample out in two dimensions. Proteins are separated according to isoelectric point (pH at which they have neutral net charge) in the first dimension, and according to their molecular weight in the second dimension.
Transfer
In order to make the proteins accessible to antibody detection, they are moved from within the gel onto a membrane made of nitrocellulose or polyvinylidene fluoride (PVDF). The membrane is placed on top of the gel, and a stack of tissue papers placed on top of that. The entire stack is placed in a buffer solution which moves up the paper by capillary action, bringing the proteins with it. Another method for transferring the proteins is called electroblotting and uses an electric current to pull proteins from the gel into the PVDF or nitrocellulose membrane. The proteins move from within the gel onto the membrane while maintaining the organization they had within the gel. As a result of this "blotting" process, the proteins are exposed on a thin surface layer for detection (see below). Both varieties of membrane are chosen for their non-specific protein binding properties (i.e. binds all proteins equally well). Protein binding is based upon hydrophobic interactions, as well as charged interactions between the membrane and protein. Nitrocellulose membranes are cheaper than PVDF, but are far more fragile and do not stand up well to repeated probings. The uniformity and overall effectiveness of transfer of protein from the gel to the membrane can be checked by staining the membrane with Coomassie or Ponceau S dyes. Coomassie is the more sensitive of the two, although Ponceau S's water solubility makes it easier to subsequently destain and probe the membrane as described below.
Blocking
Since the membrane has been chosen for its ability to bind protein, and both antibodies and the target are proteins, steps must be taken to prevent interactions between the membrane and the antibody used for detection of the target protein. Blocking of non-specific binding is achieved by placing the membrane in a dilute solution of protein - typically Bovine serum albumin (BSA) or non-fat dry milk (both are inexpensive), with a minute percentage of detergent such as Tween 20. The protein in the dilute solution attaches to the membrane in all places where the target proteins have not attached. Thus, when the antibody is added, there is no room on the membrane for it to attach other than on the binding sites of the specific target protein. This reduces "noise" in the final product of the Western blot, leading to clearer results, and eliminates false positives.
Detection
During the detection process the membrane is "probed" for the protein of interest with a modified antibody which is linked to a reporter enzyme, which when exposed to an appropriate substrate drives a colourimetric reaction and produces a colour. For a variety of reasons, this traditionally takes place in a two-step process, although there are now one-step detection methods available for certain applications.
Two step
Primary antibody
Antibodies are generated when a host species or immune cell culture is exposed to the protein of interest (or a part thereof). Normally, this is part of the immune response, whereas here they are harvested and used as sensitive and specific detection tools that bind the protein directly. After blocking, a dilute solution of primary antibody (generally between 0.5 and 5 micrograms/ml) is incubated with the membrane under gentle agitation. Typically, the solution is composed of buffered saline solution with a small percentage of detergent, and sometimes with powdered milk or BSA. The antibody solution and the membrane can be sealed and incubated together for anywhere from 30 minutes to overnight. It can also be incubated at different temperatures, with warmer temperatures being associated with more binding, both specific (to the target protein, the "signal") and non-specific ("noise").
Secondary Antibody
After rinsing the membrane to remove unbound primary antibody, the membrane is exposed to another antibody, directed at a species-specific portion of the primary antibody. This is known as a secondary antibody, and due to its targeting properties, tends to be referred to as "anti-mouse," "anti-goat," etc. Antibodies come from animal sources (or animal sourced hybridoma cultures); an anti-mouse secondary will bind to just about any mouse-sourced primary antibody. This allows some cost savings by allowing an entire lab to share a single source of mass-produced antibody, and provides far more consistent results. The secondary antibody is usually linked to biotin or to a reporter enzyme such as alkaline phosphatase or horseradish peroxidase. This means that several secondary antibodies will bind to one primary antibody and enhances the signal. Most commonly, a horseradish peroxidase-linked secondary is used in conjunction with a chemiluminescent agent, and the reaction product produces luminescence in proportion to the amount of protein. A sensitive sheet of photographic film is placed against the membrane, and exposure to the light from the reaction creates an image of the antibodies bound to the blot. As with the ELISPOT and ELISA procedures, the enzyme can be provided with a substrate molecule that will be converted by the enzyme to a colored reaction product that will be visible on the membrane . A third alternative is to use a radioactive label rather than an enzyme coupled to the secondary antibody, such as labeling an antibody-binding protein like Staphylococcus Protein A with a radioactive isotope of iodine. Since other methods are safer, quicker and cheaper this method is now rarely used.
One step
Historically, the probing process was performed in two steps because of the relative ease of producing primary and secondary antibodies in separate processes. This gives researchers and corporations huge advantages in terms of flexibility, and adds an amplification step to the detection process. Given the advent of high-throughput protein analysis and lower limits of detection, however, there has been interest in developing one-step probing systems that would allow the process to occur faster and with less consumables. This requires a probe antibody which both recognizes the protein of interest and contains a detectable label, probes which are often available for known protein tags. The primary probe is incubated with the membrane in a manner similar to that for the primary antibody in a two-step process, and then is ready for direct detection after a series of wash steps.
Analysis
After the unbound probes are washed away, the Western blot is ready for detection of the probes that are labeled and bound to the protein of interest. In practical terms, not all Westerns reveal protein only at one band in a membrane. Size approximations are taken by comparing the stained bands to that of the marker or ladder loaded during electrophoresis. The process is repeated for a structural protein, such as actin or tubulin, that should not change between samples. The amount of target protein is indexed to the structural protein to control between groups. This practice ensures correction for the amount of total protein on the membrane in case of errors or incomplete transfers.
Colorimetric detection
The colorimetric detection method depends on incubation of the Western blot with a substrate that reacts with the reporter enzyme (such as peroxidase) that is bound to the secondary antibody. This converts the soluble dye into an insoluble form of a different color that precipitates next to the enzyme and thereby stains the membrane. Development of the blot is then stopped by washing away the soluble dye. Protein levels are evaluated through densitometry (how intense the stain is) or spectrophotometry.
Chemiluminescence
Chemiluminescent detection methods depend on incubation of the Western blot with a substrate that will luminesce when exposed to the reporter on the secondary antibody. The light is then detected by photographic film, and more recently by CCD cameras which captures a digital image of the Western blot. The image is analysed by densitometry, which evaluates the relative amount of protein staining and quantifies the results in terms of optical density. Newer software allows further data analysis such as molecular weight analysis if appropriate standards are used. So-called "enhanced chemiluminescent" (ECL) detection is considered to be among the most sensitive detection methods for blotting analysis.
Radioactive detection
Radioactive labels do not require enzyme substrates, but rather allow the placement of medical X-ray film directly against the Western blot which develops as it is exposed to the label and creates dark regions which correspond to the protein bands of interest . The importance of radioactive detections methods is declining, because it is very expensive, health and safety risks are high and ECL provides a useful alternative.
Fluorescent detection
The fluorescently labeled probe is excited by light and the emission of the excitation is then detected by a photosensor such as CCD camera equipped with appropriate emission filters which captures a digital image of the Western blot and allows further data analysis such as molecular weight analysis and a quantitative Western blot analysis. Fluorescence is considered to be among the most sensitive detection methods for blotting analysis.
Secondary probing
One major difference between nitrocellulose and PVDF membranes relates to the ability of each to support "stripping" antibodies off and reusing the membrane for subsequent antibody probes. While there are well-established protocols available for stripping nitrocellulose membranes, the sturdier PVDF allows for easier stripping, and for more reuse before background noise limits experiments. Another difference is that, unlike nitrocellulose, PVDF must be soaked in 95% ethanol, isopropanol or methanol before use. PVDF membranes also tend to be thicker and more resistant to damage during use.
2-D Gel Electrophoresis
2-dimensional SDS-PAGE uses the principles and techniques outlined above. 2-D SDS-PAGE, as the name suggests, involves the migration of polypeptides in 2 dimensions. For example, in the first dimension polypeptides are separated according to isoelectric point, while in the second dimension polypeptides are separated according to their molecular weight. The isoelectric point of a given protein is determined by the relative number of positively (e.g. lysine and arginine) and negatively (e.g. glutamate and aspartate) charged amino acids, with negatively charged amino acids contributing to a high isoelectric point and positively charged amino acids contributing to a low isoelectric point. Samples could also be separated first under nonreducing conditions using SDS-PAGE and under reducing conditions in the second dimension, which breaks apart disulfide bonds that hold subunits together. SDS-PAGE might also be coupled with urea-PAGE for a 2-dimensional gel. In principle, this method allows for the separation of all cellular proteins on a single large gel. A major advantage of this method is that it often distinguishes between different isoforms of a particular protein - e.g. a protein that has been phosphorylated (by addition of a negatively charged group). Proteins that have been separated can be cut out of the gel and then analysed by mass spectrometry, which identifies the protein.
Medical diagnostic applications
The confirmatory HIV test employs a Western blot to detect anti-HIV antibody in a human serum sample. Proteins from known HIV-infected cells are separated and blotted on a membrane as above. Then, the serum to be tested is applied in the primary antibody incubation step; free antibody is washed away, and a secondary anti-human antibody linked to an enzyme signal is added. The stained bands then indicate the proteins to which the patient's serum contains antibody. A Western blot is also used as the definitive test for Bovine spongiform encephalopathy (BSE, commonly referred to as 'mad cow disease'). Some forms of Lyme disease testing employ Western blotting.
Olfactory Pathway Animation
The olfactory system is the sensory system used for olfaction. Most mammals and reptiles have two distinct parts to their olfactory system: a main olfactory system and an accessory olfactory system. The main olfactory system detects volatile, airborn substances, while the accessory olfactory system senses fluid-phase stimuli. Behavioral evidence indicates that most often, the stimuli detected by the accessory olfactory system are pheromones.



The mechanism of the olfactory system can be divided into a peripheral one, sensing an external stimulus and encoding it as an electric signal in neurons, and a central one, where all signals are integrated and processed in the central nervous system.
The olfactory system is often spoken of along with the gustatory system as the chemosensory senses because both transduce chemical signals into perception.
The mechanism of the olfactory system can be divided into a peripheral one, sensing an external stimulus and encoding it as an electric signal in neurons, and a central one, where all signals are integrated and processed in the central nervous system.
In mammals, the main olfactory system detects odorants that are inhaled through the nose, where they contact the main olfactory epithelium, which contains various olfactory receptors. These can distinguish a new odor from the background environmental odors and determine the concentration of the odor.
These olfactory receptors are connected to olfactory receptor neurons in the olfactory epithelium, which transduce receptoractivation into electrical signals in neurons. The signals travel along the olfactory nerve, which belongs to the peripheral nervous system. This nerve terminates in the olfactory bulb, which belongs to the central nervous system.
Using AutoDock
AutoDock is a suite of automated docking tools. It is designed to predict how small molecules, such as substrates or drug candidates, bind to a receptor of known 3D structure.AutoDock actually consists of two main programs: AutoDock performs the docking of the ligand to a set of grids describing the target protein; AutoGrid pre-calculates these grids.In addition to using them for docking, the atomic affinity grids can be visualised. This can help, for example, to guide organic synthetic chemists design better binders.
Autodock Docking
Extracellular signaling
Communication by extracellular signals usually involves six steps: (1) synthesis and (2) release of the signaling molecule by the signaling cell; (3) transport of the signal to the target cell; (4) detection of the signal by a specific receptor protein; (5) a change in cellular metabolism, function, or development triggered by the receptor-signal complex; and (6) removal of the signal, which often terminates the cellular response.

 Subscribe in a reader
Subscribe in a reader
How the NAD+ Works
Nicotinamide adenine dinucleotide, abbreviated NAD+, is a coenzyme found in all living cells. The compound is a dinucleotide, since it consists of two nucleotides joined through their phosphate groups: with one nucleotide containing an adenosine ring, and the other containing nicotinamide.
In metabolism, NAD+ is involved in redox reactions, carrying electrons from one reaction to another. The coenzyme is therefore found in two forms in cells: NAD+ is an oxidizing agent – it accepts electrons from other molecules and becomes reduced, this reaction forms NADH, which can then be used as a reducing agent to donate electrons. These electron transfer reactions are the main function of NAD+. However, it is also used in other cellular processes, notably as a substrate of enzymes that add or remove chemical groups from proteins, in posttranslational modifications. Due to the importance of these functions, the enzymes involved in NAD+ metabolism are targets for drug discovery.
In organisms, NAD+ can be synthesized from scratch (de novo) from the amino acids tryptophan or aspartic acid. Alternatively, components of the coenzymes are taken up from food as the vitamin called niacin. Similar compounds are released by reactions that break down the structure of NAD+. These preformed components then pass through a salvage pathway that recycles them back into the active form. Some NAD+ is also converted into nicotinamide adenine dinucleotide phosphate (NADP+); the chemistry of this related coenzyme is similar to that of NAD+, but it has different roles in metabolism.
Nicotinamide adenine dinucleotide is a dinucleotide since it consists of two nucleotides joined by a pair of bridging phosphate groups. The nucleotides consist of ribose rings, one with adenine attached to the first carbon atom (the 1' position) and the other with nicotinamide at this position. The nicotinamide group can be attached in two orientations to this anomeric carbon atom, due to these two possible structures, the compound exists as two diastereomers. It is the β-nicotinamide diastereomer of NAD+ which is found in organisms. These nucleotides are joined together by a bridge of two phosphate groups through the 5' carbons. In metabolism the compound accepts or donates electrons in redox reactions.Such reactions (summarized in formula below) involve the removal of two hydrogen atoms from the reactant (R), in the form of a hydride ion, and a proton (H+). The proton is released into solution, while the reductant RH2 is oxidized and NAD+ reduced to NADH by transfer of the hydride to the nicotinamide ring. RH2 + NAD+ → NADH + H+ + R From the hydride electron pair, one electron is transferred to the positively-charged nitrogen of the nicotinamide ring of NAD+, and the second hydrogen atom transferred to the C4 carbon atom opposite this nitrogen. The midpoint potential of the NAD+/NADH redox pair is −0.32 volts, which makes NADH a strong reducing agent. The reaction is easily reversible, when NADH reduces another molecule and is re-oxidized to NAD+. This means the coenzyme can continuously cycle between the NAD+ and NADH forms without being consumed. In appearance, all forms of this coenzyme are white amorphous powders that are hygroscopic and highly water-soluble. The solids are stable if stored dry and in the dark. Solutions of NAD+ are colorless and stable for about a week at 4 °C and neutral pH, but decompose rapidly in acids or alkalis. Upon decomposition, they form products that are enzyme inhibitors. Both NAD+ and NADH absorb strongly in the ultraviolet due to the adenine base. For example, peak absorption of NAD+ is at a wavelength of 259 nanometers (nm), with an extinction coefficient of 16,900 M-1cm-1. NADH also absorbs at higher wavelengths, with a second peak in UV absorption at 339 nm with an extinction coefficient of 6,220 M-1cm-1. This difference in the ultraviolet absorption spectra between the oxidized and reduced forms of the coenzymes at higher wavelengths makes it simple to measure the conversion of one to another in enzyme assays – by measuring the amount of UV absorption at 340 nm using a spectrophotometer. NAD+ and NADH also differ in their fluorescence. NADH in solution has an emission peak at 460 nm and a fluorescence lifetime of 0.4 nanoseconds, while the oxidized form of the coenzyme does not fluoresce. The properties of the fluorescence signal changes when NADH binds to proteins, so these changes can be used to measure dissociation constants, which are useful in the study of enzyme kinetics. These changes in fluorescence are also used to measure changes in the redox state of living cells, through fluorescence microscopy.
Monoclonal Antibody for Cancer Treatment
Ron Levy, MD, professor of Medicine at Stanford, recounts his experiences moving his discovery from the lab to the clinical setting and discusses the future of this cancer treatment. Wendy Harpham, a participant in the early clinical trials of Rituxan, the first FDA-approved monoclonal antibody for cancer treatment that Levy developed, provides a patient's perspective.
Subscribe to:
Comments (Atom)